
If the effective nuclear charge of the atom is decreased, the atomic radius of the atom is increased. If the effective nuclear charge of the atom is increased, the atomic radius is decreased. The effective nuclear charge of the atom is inversely proportional to the atomic radius.
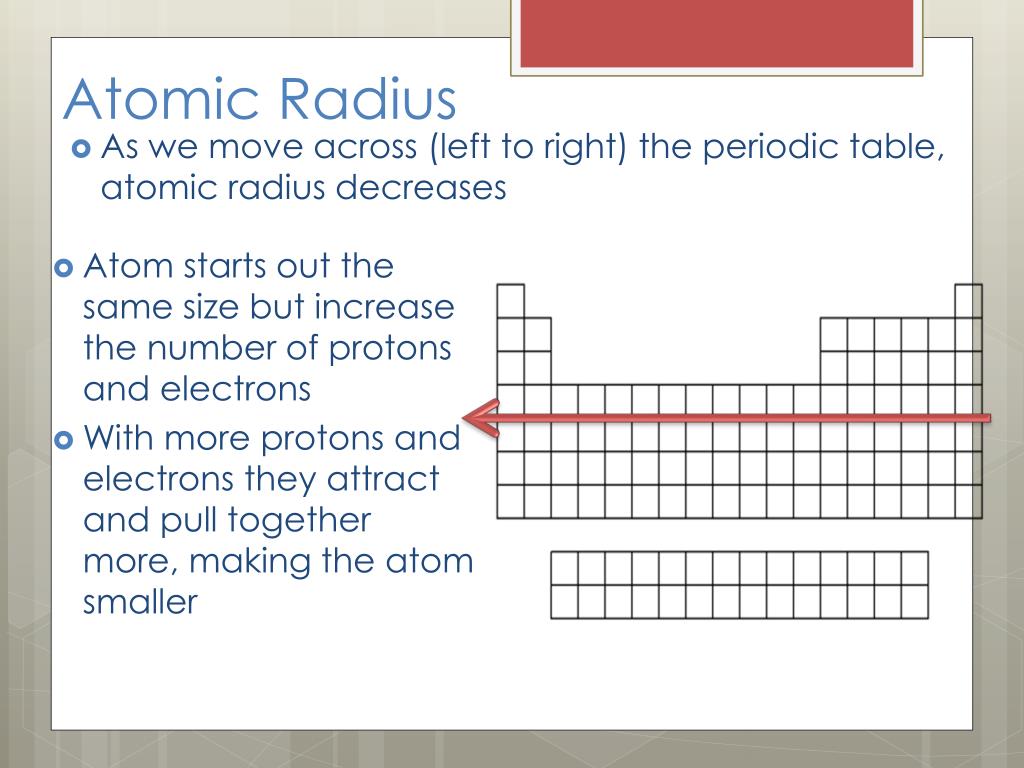
Note: We have to remember that the atomic radius of each element depends on the effective nuclear charge and shielding effect of the inner electrons in the atoms. Hence, the atomic radius of francium is largest among all the elements in the periodic table. In group trend wise, every last element in each group has the highest atomic radius in that group.Īccording to the above discussion, francium is the element present in the first element in the last period. In period trend wise, every first element in each period has the highest atomic radius in that period. In periodic table group trend wise, the atomic radius of the element in each group from top to bottom element in the group is increased. We have to remember that in periodic table period trend wise, the atomic radius of the element in each period decreases from left to right element in the period. The atomic number of francium is \ and mass number of francium is \. The atomic radius is one of the factors in this trend. In this periodic table have some periodic trends in the elements. The group is nothing but the vertical column in the periodic table. In the periodic table there are a total of eighteen groups. The period is nothing but the horizontal rows. In the periodic table there are totally seven periods.
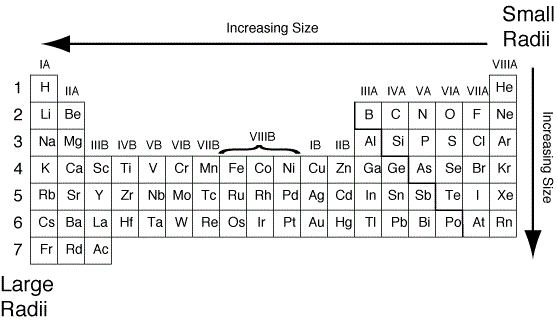
Hint: In periodic table totally \ elements.


 0 kommentar(er)
0 kommentar(er)
